Abstract
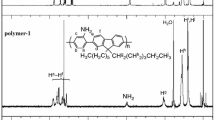
A new series of biphenylene ethynylene co-polymers, poly(2,5-dialkoxy-4-phenyleneethynylene-4,4-biphenyleneethynylene)s of the general formula [-C ≡C-4-C6H4-C6H4-4-C ≡C-C6H2(2,5-OR)2-]n (R = C4H9 P1, C8H17 P2) has been synthesized using a palladium/copper catalyzed coupling reaction between HC ≡C-4-C6H4-C6H4-4-C ≡C-H and IC6H2(2,5-OR)2I. The new co-polymer [-C ≡C-C6H2(2,5-OC8H17)2-C ≡C-C6H2(2,5-OC4H9)2-]n P5 has also been formed where different alkoxy substituents are present on alternate arene rings in the same polymer backbone. All the polymers were characterized by IR, 1H and 13C NMR spectroscopy and by GPC. The model compounds C6H5-4-C6H4-C≡C-C6H2(2,5-OR)2-4-C6H4-C6H5 (R = C4H9 M1, C8H17 M2) have also been prepared by the reaction between C6H5-4-C6H4-C≡CH and IC6H2(2,5-OR)2I. Single crystal X-ray structures of M1, M2 and Me3Si-C≡C-4-C6H4-C6H4-4-C≡C-SiMe3 were determined with a view to obtain a better understanding of the molecular and intermolecular interactions in the solid state which has been used to explain the optical properties of the polymers derived from them. The absorption and photoluminescence spectra of the polymers, P1, P2 and P5 showed that the lowest energy band is blue shifted due to the introduction of biphenylene fragments into the alkoxy substituted poly(ethynylenephenylene)s.

Synthesis, structural characterization and optical spectroscopy of poly(2,5-dialkoxy-1,4-phenylene-4-4-biphenyleneethynylene)s, [-C≡C-4-C6H4-C6H4-4-C≡C-C6H4(2,5-OR)2-]n (R = C4H9, C8H17) and their model compounds are reported.





Similar content being viewed by others
References
Pei Q B, Yu G, Zhang C Z, Yang Y and Heeger A 1995 Science 269 1086
Gunes S, Neugebaur H and Sariciftci N S 2007 Chem. Rev. 107 1324
Thomas I S W, Joly G D and Swager T M 2007 Chem. Rev. 107 1339
Mitscheke U and Bauerle P 2000 J. Mater. Chem. 10 1471
Burroughes J H, Bradley D D C, Brown A R, Marks R N, Mackay K, Friend R H, Burn P L and Holmes A B H 1990 Nature 347 539
Pang Y, Li J, Jones D, Claridge J B, Loye H C Z and Bunz U H F 1999 Macromolecules 32 4460
Zhu Z and Swager M 2002 J. Am. Chem. Soc. 124 9670
Coakley K M and Mcgehee M D 2004 Chem. Mater. 16 4533
Kim J, McQuade D T, McHugh S K and Swager T M 2000 Angew. Chem. Int. Ed. 39 3868
Zhang S-W and Swager T M 2003 J. Am. Chem. Soc. 125 3420
Bunz U H F 2000 Chem. Rev. 100 1605
Li H, Powell D R, Hayashi R K and West R 1998 Macromolecules 31 52
Ofer D, Swager T M and Wrighton M S 1995 Chem. Mater. 7 418
Pschirer N G, Miteva T, Evans U, Roberts R S, Marshall A R, Neher D, Myrick M L and Bunz U H F 2001 Chem. Mater. 13 2691
Bunz U H F 2009 Macromol. Rapid Commun. 30 772
Sonogashira K 2002 J. Organomet. Chem. 653 46
Yamamoto T, Fang Q and Morikita T2003 Macromolecules 36 4262
Bangcuyo C G, Rampey-Vaughn M E, Quan L T, Angel S M, Smith M D and Bunz U H F 2002 Macromolecules 35 1563
Weder C and Wrington M S 1996 Macromolecules 29 5157
Moroni M, Moigne J L and Luzzati S 1994 Macromolecules 27 562
Rattanatraicharoen P, Yamabuki K, Oishi T and Onimura K 2012 Polym. J. 44 224
Bunz U H F 2001 Acc. Chem. Res. 34 998
Younus M, Köhler A, Cron S, Chawdhury N, Al-Mandhary M R A, Lewis J, Long N J, Friend R H and Raithby P R 1998 Angew. Chem. Int. Ed. 37 3036
Chawdhury N, Köhler A K, Friend R H, Younus M, Long N J, Raithby P R and Lewis J 1998 Macromolecules 31 722
Long N J, White A J P, Williams D J and Younus M 2002 J. Organomet. Chem. 649 94
Saha R, Qaium M A, Debnath D, Younus M, Chawdhury N, Sultana N, Kociok-Kohn G, Ooi L and Raithby P R 2005 Dalton Trans. 2760
Takahashi S, Kuroyama Y, Sonogahara K and Hagihara N 1980 Synthesis 627
Antonelli E, Rosi P, Sterzo C L and Viola E 1999 J. Organomet. Chem. 578 210
Bao Z, Chen Y, Cai R and Yu L 1993 Macromolecules 26 5281
Errington R J 1997 In Advanced Practical Inorganic and Metalorganic Chemistry (London: Chapman and Hall)
Otwinowski Z and Minor W 1999 In DENZO-SMN Manual (Dallas: University of Texas Southwestern Medical Center)
Altomare A, Burla M C, Camalli M, Cascarano G L, Giacovazzo C, Guagliardi A, Moliterni A G G, Polidori G and Spagna R 1999 J. Appl. Cryst. 32 115
Sheldrick G M 1997 SHELXL 97 Program for Crystal Structure Determination, (Germany: Universität Göttingen)
Farrugia L J 1997 J. Appl. Cryst. 30 565
Pizzoferrato R, Berliocchi M, Di Carlo A, Lugli P, Venanzi M, Micozzi A, Ricci A and Lo Sterzo C 2003 Macromolecules 36 2215
Perera K P U, Krawiec M and Smith D W 2002 Tetrahedron 58 10197
Perera K P U, Abboud K A, Smith D W and Krawiec M 2003 Acta Cryst. C 59 107
Allen F H 2002 Acta Cryst. Sec B 58 380
Levitus M, Schmieder K, Ricks H, Shimizu K D, Bunz U H F and Garcia-Garibay M A 2001 J. Am. Chem. Soc. 123 4259
James P V, Sudeep P K, Suresh C H and Thomas K G 2006 J. Phys. Chem. A 110 4329
Francke V, Mangel T and Mullen K 1998 Macromolecules 31 2447
Köhler A, Wilson J S, Friend R H, Al-Suti M K, Khan M S, Gerhard A and Bassler H 2002 J. Chem. Phys. 116 9457
Chawdhury N, Köhler A K, Friend R H, Wong W-Y, Lewis J, Younus M, Raithby P R, Corcoran T C, Al-Mandhary M R A and Khan M S 1999 J. Chem. Phys. 110 4963
Acknowledgements
We are grateful to the Leverhulme Trust and the Royal Society, UK; and Higher Education Quality Enhancement Project (HEQEP), UGC, Bangladesh for financial support (to M. Y.), Shahjalal University for study leave (to M. Y.), and the EPSRC for funding to purchase the X-ray diffractometer. The EPSRC sponsored polymer molecular weight determination service run by RAPRA is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The 13C NMR spectrum of C6H5-4-C6H4-C≡C-C6H4(2,5-OC8H17)2-C6H4-4-C6H5 M2; and crystal data, data collection and structure refinement for M1, M2 and Me3SiC≡C(C6H4)2C≡CSiMe3 are available from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336 033; or e-mail: deposit@ccdc.cam.ac.uk. The CCDC reference numbers for the three crystal structures are 997486-997488. Supplementary information is also available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
NAG, O.K., ANIS-UL-HAQUE, K.M., DEBNATH, D. et al. Synthesis and optical properties of biphenylene ethynylene co-polymers and their model compounds. J Chem Sci 127, 365–374 (2015). https://doi.org/10.1007/s12039-015-0789-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0789-y