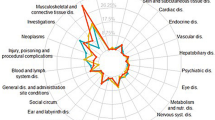
Abstract
Background
Malignant skin tumors are adverse events of concern regarding Janus kinase (JAK) inhibitors.
Aim
This study aimed to evaluate the association between JAK inhibitors and adverse events of malignant skin tumors, and to characterize the main features.
Method
Data (2012–2021) were collected using the US Food and Drug Administration Adverse Event Reporting System (FAERS). Adverse event cases of JAK inhibitors as the primary suspected drug were extracted for further analysis. Disproportionality analysis evaluated the association between JAK inhibitors and malignant skin tumor events by estimating the reporting odds ratio (ROR) and the information component (IC) with 95% confidence intervals (95% CI).
Results
A total of 142,673 cases with JAK inhibitors as a primary suspected drug were collected, including 1400 malignant skin tumor events. Ruxolitinib, upadacitinib, tofacitinib, and baricitinib were included in the disproportionality analysis. Three JAK inhibitors were associated with malignant skin tumor events, namely ruxolitinib (ROR 5.40, 95% CI 5.03–5.81; IC 2.39, 95% CI 2.14–2.62), upadacitinib (ROR 4.79, 95% CI 4.03–5.71; IC 2.24, 95% CI 1.62–2.77), and tofacitinib (ROR 1.67, 95% CI 1.53–1.83; IC 0.73, 95% CI 0.43–1.02). The median time to onset time was 378.5 days.
Conclusion
We found association between malignant skin tumors and ruxolitinib, upadacitinib, and tofacitinib. More attention should be paid to these events when prescribing JAK inhibitors in clinical practice.



Similar content being viewed by others
References
Baş S, Cakir S, Ertas Y, et al. Epidemiological evaluation of non-melanoma skin cancer ac- cording to body distribution. Turk Arch Dermatol Venerol/Turkderm. 2020;54:51–7.
MacFarlane LA, Todd DJ. Kinase inhibitors: the next generation of therapies in the treatment of rheumatoid arthritis. Int J Rheum Dis. 2014;17:359–68.
Samadi A, Nasrollahi SA, Hashemi A, et al. Janus kinase (JAK) inhibitors for the treat- ment of skin and hair disorders: a review of literature. J Dermatol Treat. 2017;28(6):476–83.
Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15(1):23.
Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67(3):616–25.
Charles-Schoeman C, Gonzalez-Gay MA, Kaplan I, et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin Arthritis Rheum. 2016;46(1):71–80.
Xeljanz (tofacitinib): highlights of prescribing information. New York: Pfizer, 2020 (package insert).
Bas S, Cakir S, Ertas Y, et al. Epidemiological evaluation of non-melanoma skin cancer according to body distribution. Turkderm-Turk Archiv Dermatol Venerol. 2020;54(2):51–7.
Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003.
Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–26.
Winthrop KL, Cohen SB. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol. 2022;18(5):301–4.
Malato A, Rossi E, Palumbo GA, et al. Drug-related cutaneous adverse events in Philadelphia chromosome-negative myeloproliferative neoplasms: a literature review. Int J Mol Sci. 2020;21(11):3990.
Fabiano A, Calzavara-Pinton P, Monari P, et al. Eruptive squamous cell carcinomas with keratoacanthoma-like features in a patient treated with ruxolitinib. Br J Dermatol. 2015;173(4):1098–9.
Khanna U, Richardson V, Hexner E, et al. A photo-distributed papulopustular eruption and multiple squamous cell carcinomas in a patient on ruxolitinib. JAAD Case Rep. 2019;5(10):895–7.
Dunaway S, Yu Y, Neltner S. Development of aggressive squamous cell carcinoma with perineural invasion during ruxolitinib treatment. Dermatol Surg. 2019;45(5):734–6.
Aboul-Fettouh N, Nijhawan RI. Aggressive squamous cell carcinoma in a patient on the Janus kinase inhibitor ruxolitinib. JAAD Case Rep. 2018;4(5):455–7.
Aleisa AI, Plante JG, Hsia LB. A case of aggressive squamous cell carcinoma with lymphovascular invasion during treatment with the Janus kinase inhibitor tofacitinib. JAAD Case Rep. 2020;6(8):727–30.
Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–12.
Fleischmann RM, Genovese MC, Enejosa JV, et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis. 2019;78(11):1454–62.
Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–24.
Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303–11.
van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and Safety of Upadacitinib Monotherapy in Methotrexate-Naive Patients With Moderately-to-Severely Active Rheumatoid Arthritis (SELECT-EARLY): A Multicenter, Multi-Country, Randomized, Double-Blind. Active Comparator-Controlled Trial Arthritis Rheumatol. 2020;72(10):1607–20.
Verstovsek S, Gotlib J, Mesa RA, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017;10(1):156.
Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101(7):821–9.
Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173(4):949–61.
Greif CS, Srivastava D, Nijhawan RI. Janus Kinase inhibitors and non-melanoma skin cancer. Curr Treat Options Oncol. 2021;22(2):11.
Olivera PA, Lasa JS, Bonovas S, et al. Safety of Janus Kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554-1573.e12.
Curtis JR, Lee EB, Martin G, et al. Analysis of non-melanoma skin cancer across the tofacitinib rheumatoid arthritis clinical programme. Clin Exp Rheumatol. 2017;35(4):614–22.
Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76(7):1253–62.
Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17(8):1541–50.
Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21(1):89.
Nash P, Coates LC, Kivitz AJ, et al. Safety and efficacy of tofacitinib in patients with active psoriatic arthritis: interim analysis of OPAL balance, an open-label. Long-Term Extension Study Rheumatol Ther. 2020;7(3):553–80.
Genovese MC, Kalunian K, Gottenberg JE, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322(4):315–25.
Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis. 2017;76(6):1009–19.
Westhovens R, Taylor PC, Alten R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis. 2017;76(6):998–1008.
Clarke B, Yates M, Adas M, et al. The safety of JAK-1 inhibitors. Rheumatology. 2021;60(Suppl 2):ii24–30.
Wu B, Luo M, Wu F, et al. Acute kidney injury associated with remdesivir: a comprehensive pharmacovigilance analysis of COVID-19 reports in FAERS. Front Pharmacol. 2022;13:692828.
Wu B, Hu Q, Tian F, et al. A pharmacovigilance study of association between proton pump inhibitor and dementia event based on FDA adverse event reporting system data. Sci Rep. 2021;11(1):10709.
Wu L, Ingle T, Liu Z, et al. Study of serious adverse drug reactions using FDA-approved drug labeling and MedDRA. BMC Bioinformatics. 2019;20(Suppl 2):97.
van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Jedlowski PM. Association of non-melanoma skin cancers, melanoma and merkel cell carcinoma with dermatologic medications, a case control pharmacovigilance study of the FDA Adverse Events Reporting System. Dermatology. 2023 Apr 13.
Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. Adv Exp Med Biol. 2020;1268:123–39.
Herrera AP, Snipes SA, King DW, et al. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;100(Suppl 1):S105-12.
Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14.
Neha R, Subeesh V, Beulah E, et al. Existence of Notoriety Bias in FDA Adverse Event Reporting System Database and Its Impact on Signal Strength. Hosp Pharm. 2021;56(3):152–8.
Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis. 2022;81(3):335–43.
Simon TA, Thompson A, Gandhi KK, et al. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17(1):212.
Tegtmeyer K, Ravi M, Zhao J, et al. Off-label studies on the use of ruxolitinib in dermatology. Dermatitis. 2021;32(3):164–72.
Johnson NM, Prickett KA, Phillips MA. Systemic medications linked to an increased risk for skin malignancy. Cutis. 2019;104(4):E32-e36.
Samadi A, Ahmad Nasrollahi S, Hashemi A, et al. Janus kinase (JAK) inhibitors for the treatment of skin and hair disorders: a review of literature. J Dermatolog Treat. 2017;28(6):476–83.
Acknowledgements
This research was supported by National Key Clinical Specialties Construction Program.
Funding
This study was supported by the 1·3·5 projects for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2018HXFH050) of the National Key Clinical Specialties Construction Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, T., Gao, R., Li, L. et al. Analysis of the association between Janus kinase inhibitors and malignant skin tumors using the Food and Drug Administration Adverse Event Reporting System. Int J Clin Pharm 45, 1483–1491 (2023). https://doi.org/10.1007/s11096-023-01634-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01634-5