Abstract
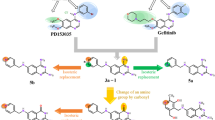
New quinoline derivatives 6, 7 and 19, pyrimidoquinoline derivatives 8–16 and triazolopyrimidoquinoline derivatives 17 and 18 bearing a bromo-substituent were synthesized starting from 3-(4-Bromophenylamino)-5,5-dimethylcyclohex-2-enone 3. All the newly synthesized compounds were evaluated for their in vitro anticancer activity against human breast cancer cell line (MCF7). Compounds 9, 11, 17 and 18 showed IC50 values (36.4, 39.7, 39.02 and 36.4 μM, respectively) comparable to that of the reference drug doxorubicin (IC50 = 32.02 μM). On the other hand, compound 6, 14 and 19 exhibited better activity than doxorubicin with IC50 values of 8.5, 23.5 and 23.7 μM. Additionally, the most potent compounds 6, 14 and 19 were evaluated for their ability to enhance the cell killing effect of γ-radiation.
Similar content being viewed by others
References
Abouzid, K. and Shouman, S., Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg. Med. Chem., 16, 7543–7551 (2008).
Al-Ghamdi, M. A., Abd El-Wahab, H. F., Mohamed, M. H., and El-Agrody, M. A., Synthesis and antitumor activities of 4H-pyrano[3,2-h]quinoline-3-carbonitrile, 7H-pyrimido [4′,5′:6,5]pyrano[3,2-h]quinoline, and 14H-pyrimido[4′,5′: 6,5]pyrano[3,2-h][1,2,4]triazolo[1,5-c]quinoline derivatives. Lett. Drug Des. Discov., 9, 459–470 (2012).
Alqasoumi, S. I., Al-Taweel, A. M., Alafeefy, A. M., Noaman, E., and Ghorab, M. M., Novel quinolines and pyrimido[4, 5-b]quinolines bearing biologically active sulfonamide moiety as a new class of antitumor agents. Eur. J. Med. Chem., 45, 738–744 (2010).
Alqasoumi, S. I., Al-Taweel, A. M., Alafeefy, A. M., Hamed, M. M., Noaman, E., and Ghorab, M. M., Synthesis and biological evaluation of 2-amino-7,7-dimethyl 4-substituted-5-oxo-1-(3,4,5-trimethoxy)-1,4,5,6,7,8-hexahydro-quinoline-3-carbonitrile derivatives as potential cytotoxic agents. Bioorg. Med. Chem. Lett., 19, 6939–6942 (2009).
Amr, A. G., Mohamed, A. M., Mohamed, S. F., Abdel-Hafez, N. A., and Hammam, Ael-F., Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg. Med. Chem., 14, 5481–5488 (2006).
Behforouz, M., Cai, W., Mohammadi, F., Stocksdale, M. G., Gu, Z., Ahmadian, M., Baty, D. E., Etling, M. R., Al-Anzi, C. H., Swiftney, T. M., Tanzer, L. R., Merriman, R. L., and Behforouz, N. C., Synthesis and evaluation of antitumor activity of novel N-acyllavendamycin analogues and quinoline-5,8-diones. Bioorg. Med. Chem., 15, 495–510 (2007).
Chauhan, P. M., Martins, C. J., and Horwell, D. C., Syntheses of novel heterocycles as anticancer agents. Bioorg. Med. Chem., 13, 3513–3518 (2005).
Cheng, Y., An, L. K., Wu, N., Wang, X. D., Bu, X. Z., Huang, Z. S., and Gu, L. Q., Synthesis, cytotoxic activities and structure-activity relationships of topoisomerase I inhibitors: indolizinoquinoline-5,12-dione derivatives. Bioorg. Med. Chem., 16, 4617–4625 (2008).
Cocco, M. T., Congiu, C., Lilliu, V., and Onnis, V., Synthesis and in vitro antitumoral activity of new hydrazinopyrimidine-5-carbonitrile derivatives. Bioorg. Med. Chem., 14, 366–372 (2006).
Dogan, H. N., Duran, A., and Rollas, S., Synthesis and preliminary anticancer activity of new 1H-4,5-dihydro-3-(3-hydroxy-2-naphthyl)-4-substituted-1,2,4-triazoline-5-thiones. Indian J. Chem., 44, 2301–2307 (2005).
El-Sayed, O. A., Al-Turki, T. M., Al-Daffiri, H. M., Al-Bassam, B. A., and Hussein, M. E., Tetrazolo[1,5-a] quinoline derivatives as anti-inflammatory and antimicrobial agents [1]. Boll. Chim. Farm., 143, 227–238 (2004).
Eswaran, S., Adhikari, A. V., and Ajay Kumar, R., New 1,3-oxazolo[4,5-c]quinoline derivatives: synthesis and evaluation of antibacterial and antituberculosis properties. Eur. J. Med. Chem., 45, 957–966 (2010).
Ferlin, M. G., Gatto, B., Chiarelotto, G., and Palumbo, M., Novel pyrrolo[3,2-f]quinolines: synthesis and antiproliferative activity. Bioorg. Med. Chem., 9, 1843–1848 (2001).
Ghorab, M. M., Ragab, F. A., Heiba, H. I., and Ghorab, W. M., Design and synthesis of some novel quinoline derivatives as anticancer and radiosensitizing agents targeting VEGFR tyrosine kinase. J. Heterocycl. Chem., 48, 1269–1279 (2011).
Gopal, M., Shenoy, S., and Doddamani, L. S., Antitumor activity of 4-amino and 8-methyl-4-(3-diethylamino propylamino) pyrimido[4′,5′:4,5]thieno (2,3-b) quinolines. J. Photochem. Photobiol., 72, 7543–7551 (2007).
Heiniger, B., Gakhar, G., Prasain, K., Hua, D. H., and Nguyen, T. A., Second-generation substituted quinolines as anticancer drugs for breast cancer. Anticancer Res., 30, 3927–3932 (2010).
Joshi, A. A., Narkhede, S. S., and Viswanathan, C. L., Design, synthesis and evaluation of 5-substituted amino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolines as novel antimalarials. Bioorg. Med. Chem. Lett., 15, 73–76 (2005).
Kamen, B. A., Cole, P. D., and Bertino, J. R., Chemotherapeutic agents. In Cancer Medicine, Bast, R. C., Kufe, D. W., Pollock, R. E., Weichselbaum, R. R., Holland Frei, J. F., and BCDecke, E. (Eds). 5, 612–705 (2000).
Kashyap, S. J., Sharma, P. K., Garg, V. K., Dudhe, R., and Kumar, N., Review on synthesis and various biological potential of thiazolopyrimidine derivatives. J. Adv. Sci. Res., 2, 18–24 (2011).
Kategaonkar, A. H., Pokalwar, R. U., Sonar, S. S., Gawali, V. U., Shingate, B. B., and Shingare, M. S., Synthesis, in vitro antibacterial and antifungal evaluations of new alpha-hydroxyphosphonate and new alpha-acetoxyphosphonate derivatives of tetrazolo [1,5-a] quinoline. Eur. J. Med. Chem., 45, 1128–1132 (2010).
Kemnitzer, W., Kuemmerle, J., Jiang, S., Zhang, H. Z., Sirisoma, N., Kasibhatla, S., Crogan-Grundy, C., Tseng, B., Drewe, J., and Cai, S. X., Discovery of 1-benzoyl-3-cyanopyrrolo[1,2-a]quinolines as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. Part 1: Structure-activity relationships of the 1- and 3-positions. Bioorg. Med. Chem. Lett., 18, 6259–6264 (2008).
Kim, Y. H., Shin, K. J., Lee, T. G., Kim, E., Lee, M. S., Ryu, S. H., and Suh, P. G., G2 arrest and apoptosis by 2-amino-N-quinoline-8-yl-benzenesulfonamide (QBS), a novel cytotoxic compound. Biochem. Pharmacol., 69, 1333–1341 (2005).
Mahmoudian, M. and Rahimi-Moghaddam, P., The anticancer activity of noscapine: a review. Recent Pat. Anticancer Drug Discov., 4, 92–97 (2009).
Mani, S., Macapinlac, M., Jr., Goel, S., Verdier-Pinard, D., Fojo, T., Rothenberg, M., and Colevas, D., The clinical development of new mitotic inhibitors that stabilize the microtubule. Anticancer Drugs, 15, 553–558 (2004).
Metwally, K., Khalil, A., Pratsinis, H., and Kletsas, D., Synthesis, in-vitro cytotoxicity, and a preliminary structureactivity relationship investigation of pyrimido[4,5-c]quinoline-1(2H)-ones. Arch. Pharm. (Weinheim), 343, 465–472 (2010).
Milner, E., McCalmont, W., Bhonsle, J., Caridha, D., Carroll, D., Gardner, S., Gerena, L., Gettayacamin, M., Lanteri, C., Luong, T., Melendez, V., Moon, J., Roncal, N., Sousa, J., Tungtaeng, A., Wipf, P., and Dow, G., Structure-activity relationships amongst 4-position quinoline methanol antimalarials that inhibit the growth of drug sensitive and resistant strains of Plasmodium falciparum. Bioorg. Med. Chem. Lett., 20, 1347–1351 (2010).
Mulvihill, M. J., Ji, Q. S., Coate, H. R., Cooke, A., Dong, H., Feng, L., Foreman, K., Rosenfeld-Franklin, M., Honda, A., Mak, G., Mulvihill, K. M., Nigro, A. I., O’Connor, M., Pirrit, C., Steinig, A. G., Siu, K., Stolz, K. M., Sun, Y., Tavares, P. A., Yao, Y., and Gibson, N. W., Novel 2-phenylquinolin-7-yl-derived imidazo[1,5-a]pyrazines as potent insulin-like growth factor-I receptor (IGF-IR) inhibitors. Bioorg. Med. Chem., 16, 1359–1375 (2008).
Nishii, H., Chiba, T., Morikami, K., Fukami, T. A., Sakamoto, H., Ko, K., and Koyano, H., Discovery of 6-benzyloxyquinolines as c-Met selective kinase inhibitors. Bioorg. Med. Chem. Lett., 20, 1405–1409 (2010).
Nishimura, Y., Rationale for chemoradiotherapy. Int. J. Clin. Oncol., 9, 414–420 (2004).
Palaska, E., Sahin, G., Kelicen, P., Durlu, N. T., and Altinok, G., Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco, 57, 101–107 (2002).
Palit, P., Paira, P., Hazra, A., Banerjee, S., Gupta, A. D., Dastidar, S. G., and Mondal, N. B., Phase transfer catalyzed synthesis of bis-quinolines: antileishmanial activity in experimental visceral leishmaniasis and in vitro antibacterial evaluation. Eur. J. Med. Chem., 44, 845–853 (2009).
Pannala, M., Kher, S., Wilson, N., Gaudette, J., Sircar, I., Zhang, S. H., Bakhirev, A., Yang, G., Yuen, P., Gorcsan, F., Sakurai, N., Barbosa, M., and Cheng, J. F., Synthesis and structure-activity relationship of 4-(2-aryl-cyclopropylamino)-quinoline-3-carbonitriles as EGFR tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett., 17, 5978–5982 (2007).
Singhal, N., Sharma, P. K., Dudhe, R., and Kumar, N., Recent advancement of triazole derivatives and their biological significance. J. Chem. Pharm. Res., 3, 126–133 (2011).
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S., and Boyd, M. R., New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst., 82, 1107–1112 (1990).
Zhao, Y. L., Chen, Y. L., Chang, F. S., and Tzeng, C. C., Synthesis and cytotoxic evaluation of certain 4-anilino-2-phenylquinoline derivatives. Eur. J. Med. Chem., 40, 792–797 (2005).
Zhou, J., Gupta, K., Aggarwal, S., Aneja, R., Chandra, R., Panda, D., and Joshi, H. C., Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol. Pharmacol., 63, 799–807 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorab, M.M., Ragab, F.A., Heiba, H.I. et al. Novel brominated quinoline and pyrimidoquinoline derivatives as potential cytotoxic agents with synergistic effects of γ-radiation. Arch. Pharm. Res. 35, 1335–1346 (2012). https://doi.org/10.1007/s12272-012-0803-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-0803-6